Clinical Studies
Human Clinical Study Design
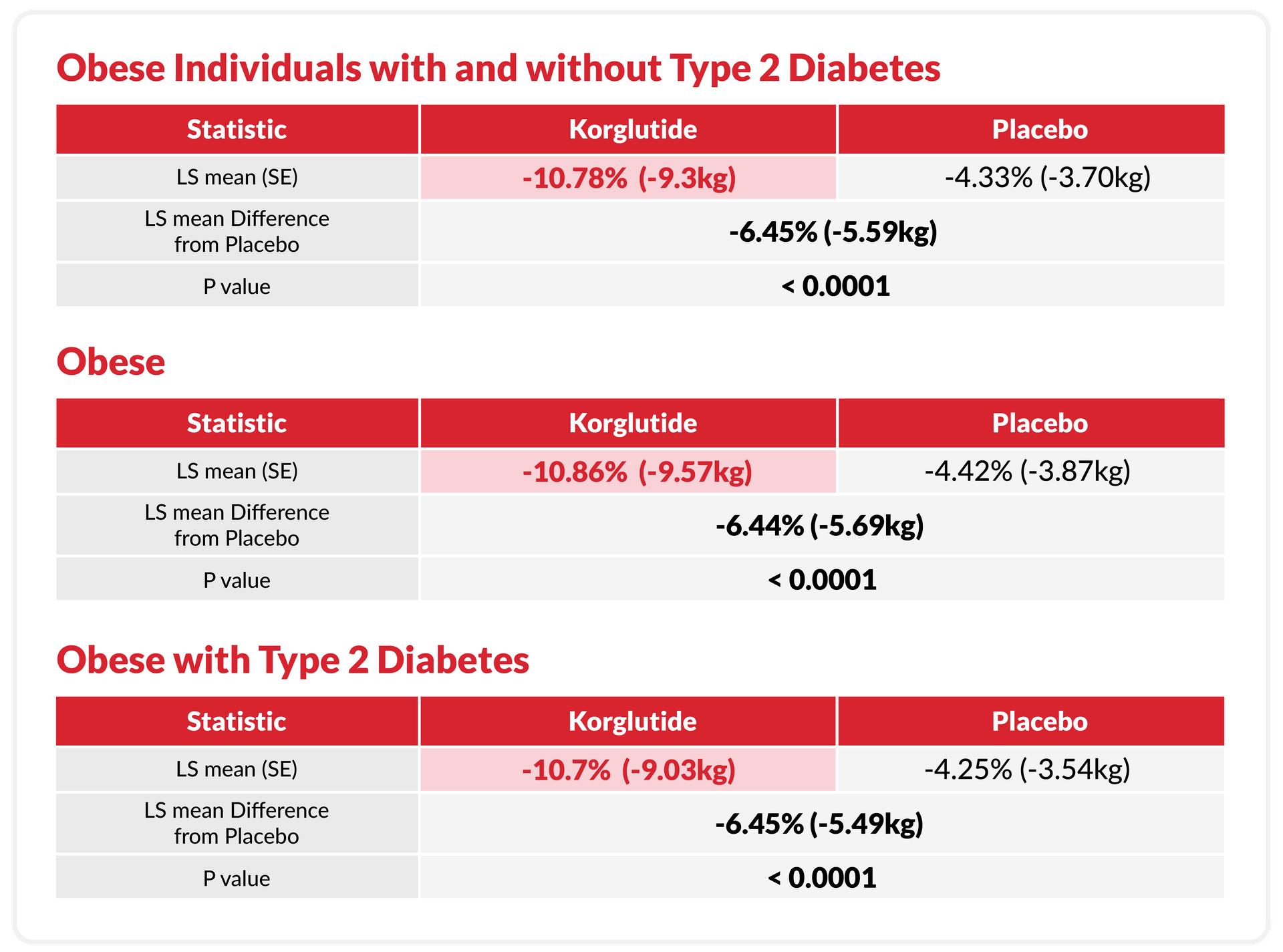
A Randomized, Double-Blind, Placebo-Controlled Phase III Study to Assess the Efficacy and Safety of Korglutide in Weight Management in Obese Individuals with and without Type 2 Diabetes.
▪ Subject: Non-diabetic cohort: BMI ≥30 kg/m² / Diabetic cohort (Type 2 Diabetes):
BMI ≥27 kg/m².
▪ Number: Placebo: 50, Korglutide:50.
▪ Statistical analysis: One-way ANOVA /Dunnett's multiple comparisons test.
▪ Period: 12-week.
▪ Dosage: a daily oral dose of 100 mg.
▪
Diet restriction: 500 kcal/day, 30 min light walking (recommended).
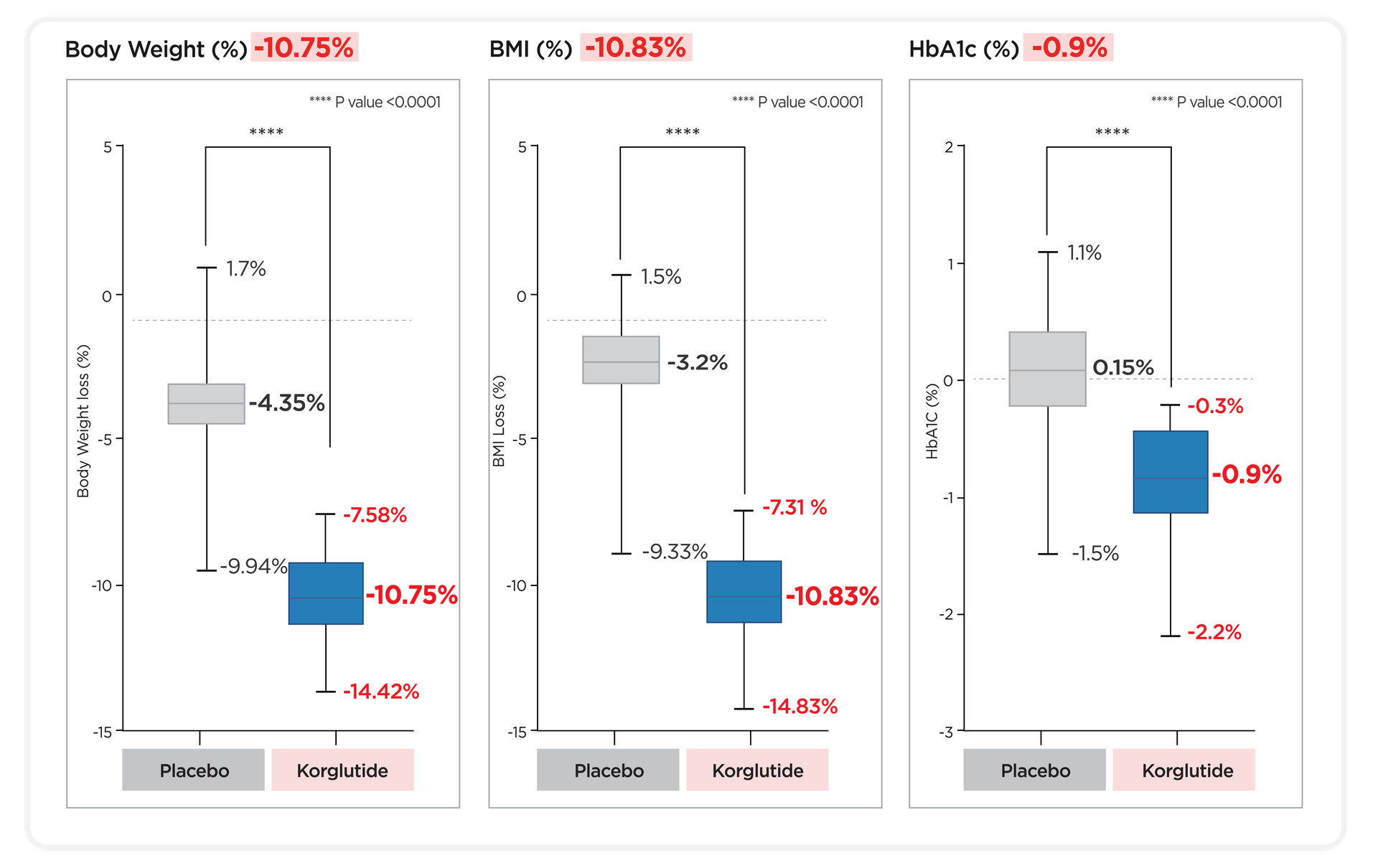
The percentage reduction in body weight from baseline to week 12.
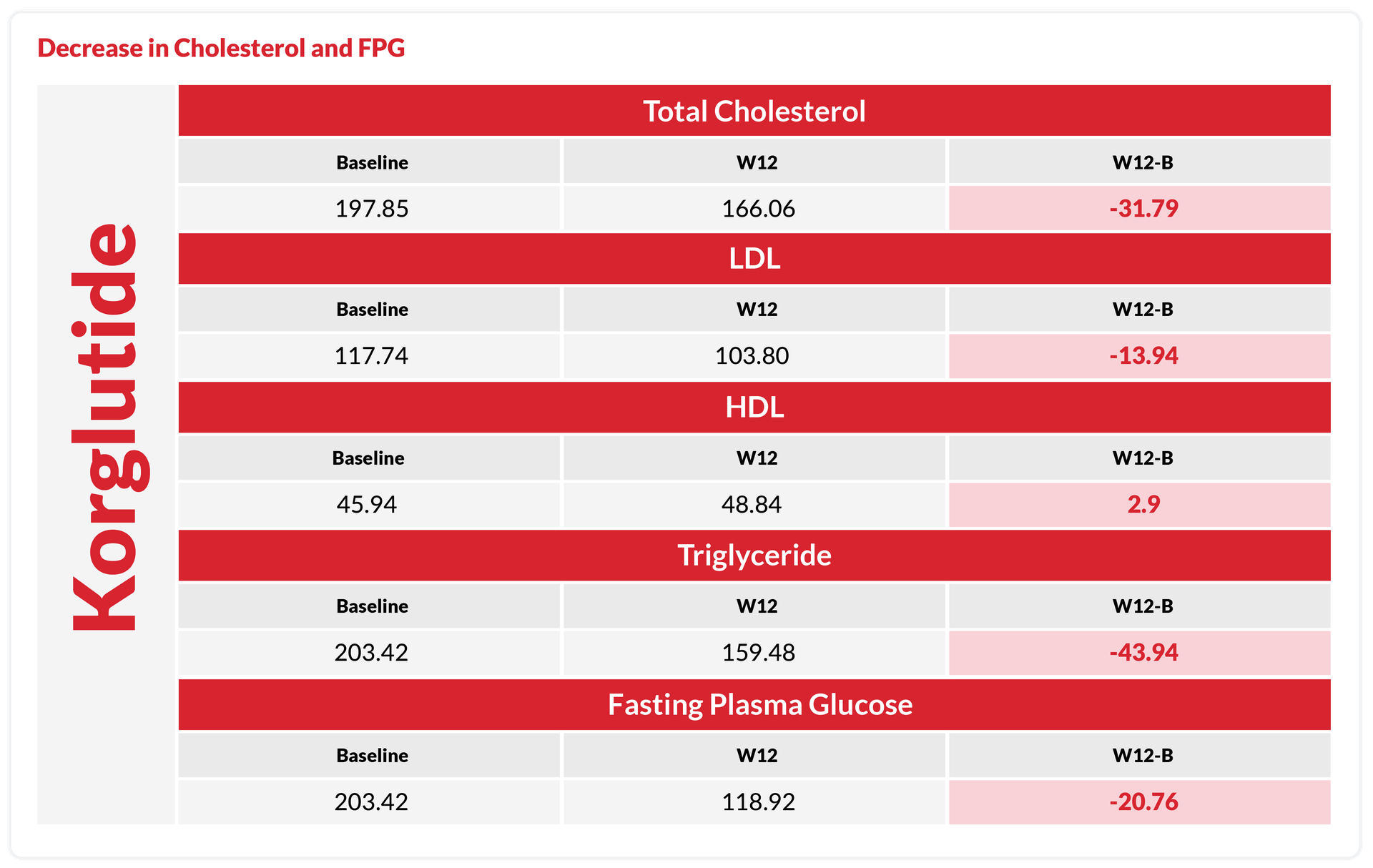
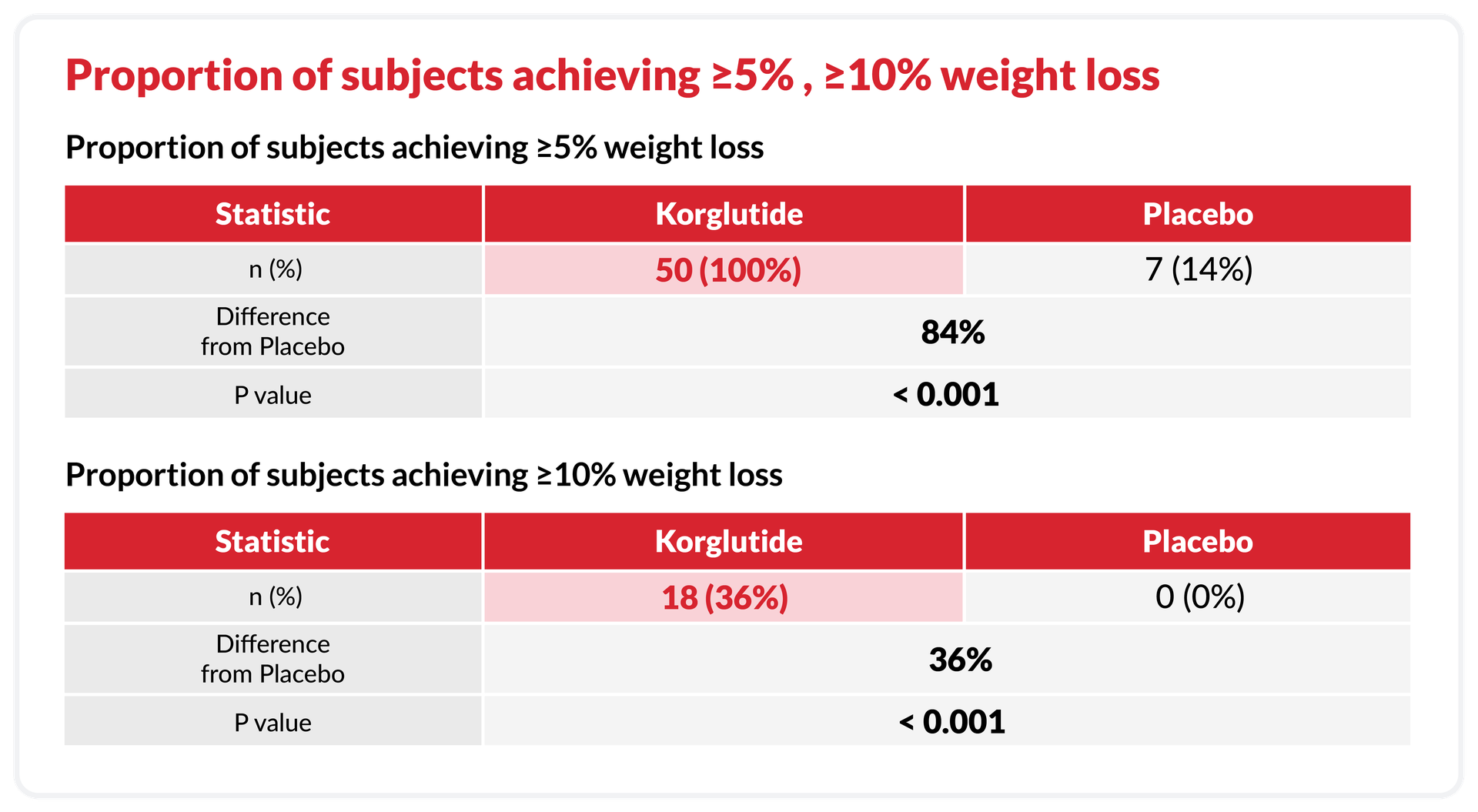
Included changes in body mass index (BMI), body fat mass, skeletal muscle mass, HbA1c, and lipid profile (including cholesterol, LDL, HDL, triglycerides), as well as the proportion of subjects achieving ≥5% and ≥10% weight loss.
Human Clinical Study Results for Weight Loss
Body Weight Loss: -10.78% in 12 weeks Statistical significance: p < 0.0001
Human Clinical Study Results for Weight Loss
Clinically significant improvements in Weight, BMI, and HbA1c.
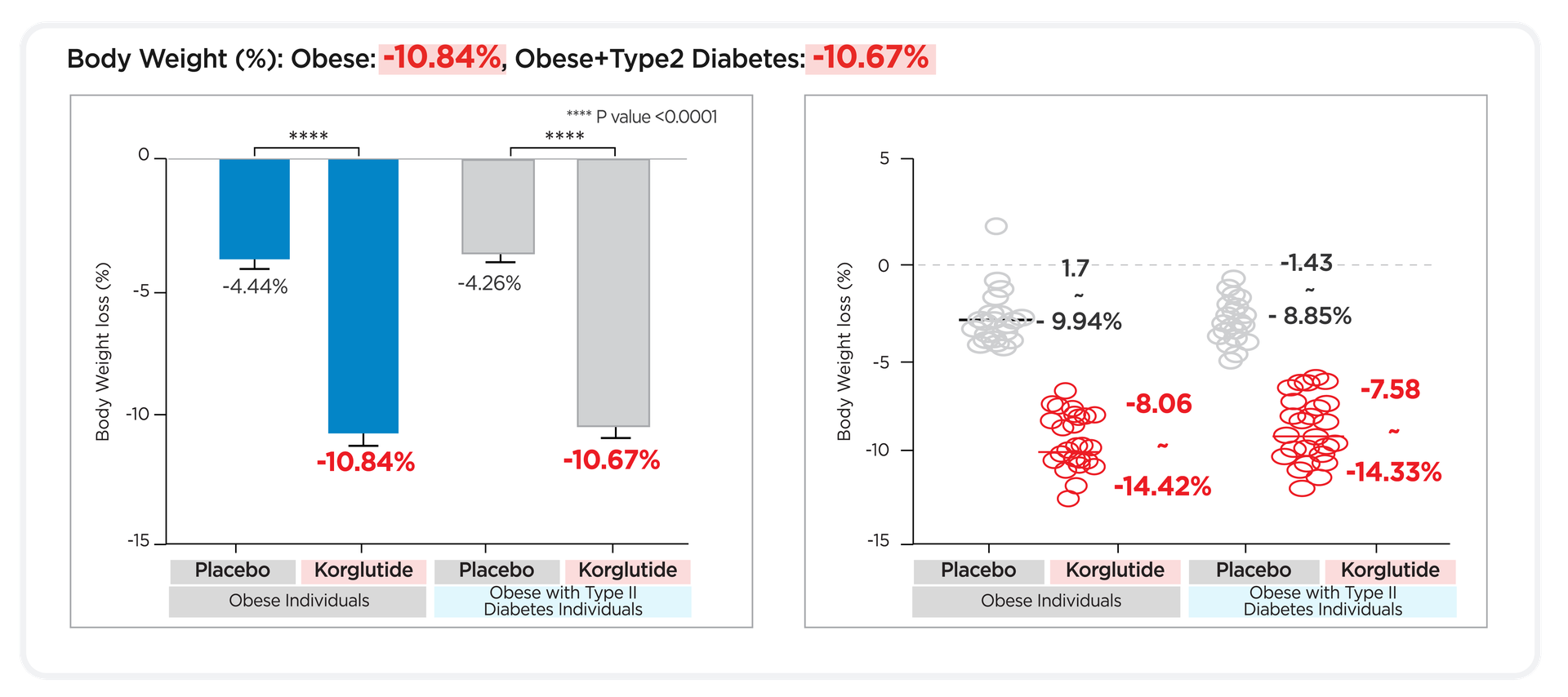
Korglutide
works regardless of disease status
Proving its role as a universal, safe, and effective weight loss solution.
100% Responders, 36% High Achievers
Korglutide Defines
Weight Loss Success. Clinically Superior to Placebo.
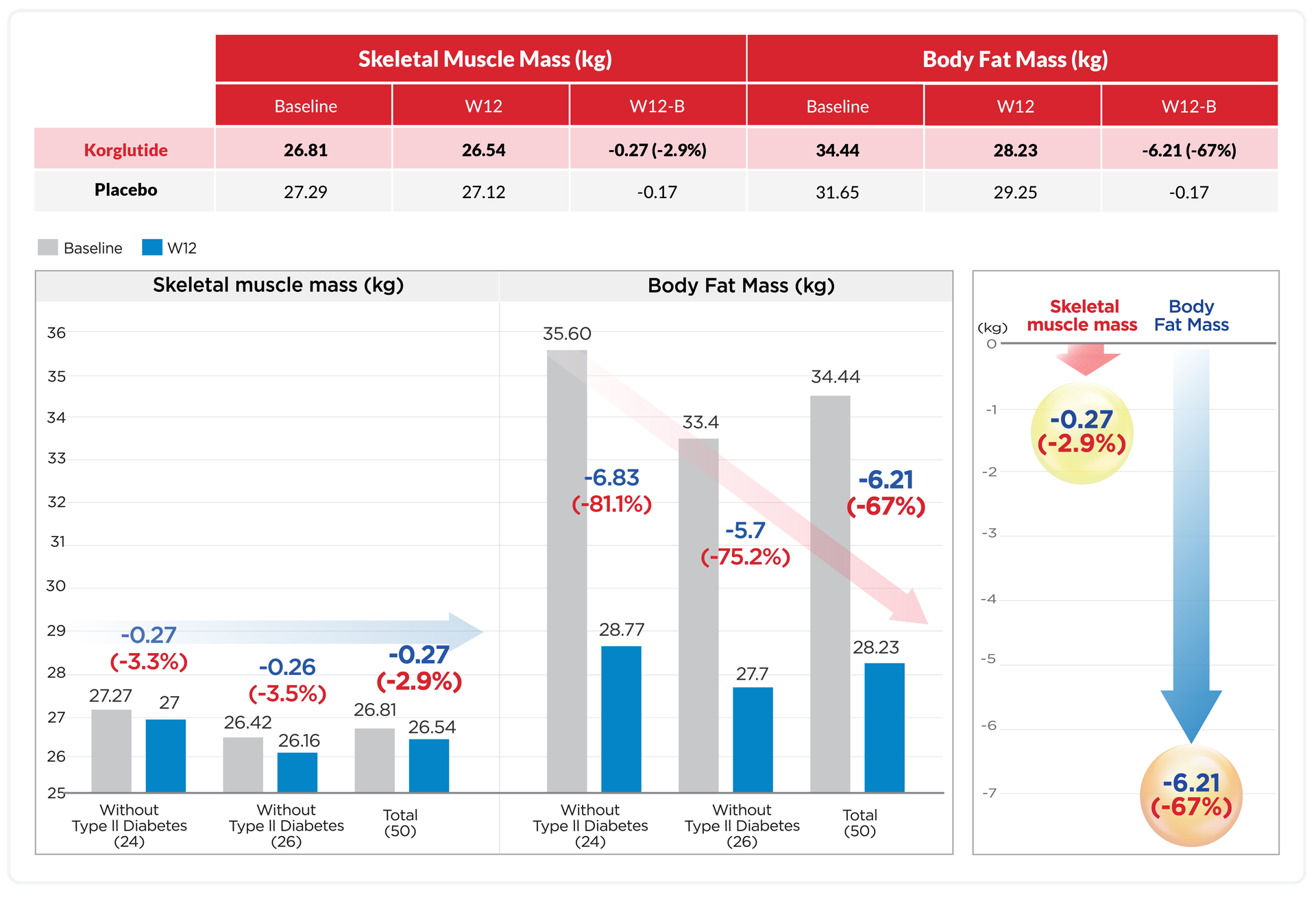
Minimize Skeletal Muscle Mass loss
Maximize Fat mass loss
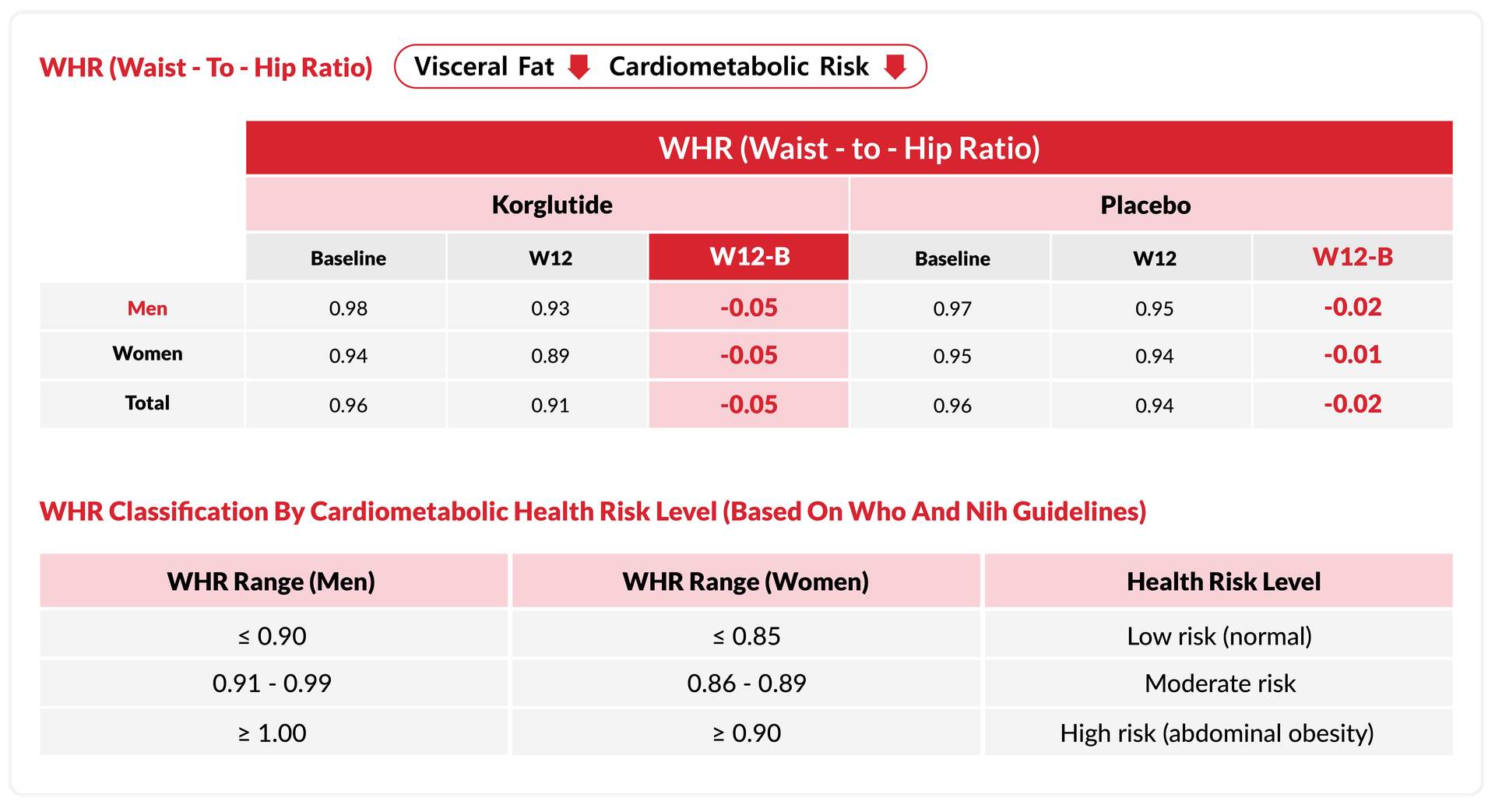
Korglutide Significantly Improves
Waist
to
Hip Ratio (WHR)
Korglutide demonstrated a consistent reduction of 0.05 in WHR for both men and women over 12 weeks
Indicating meaningful improvements in visceral fat and cardiometabolic health risk.
Key Metabolic Improvements with Korglutide
Decrease in Cholesterol & FPG